
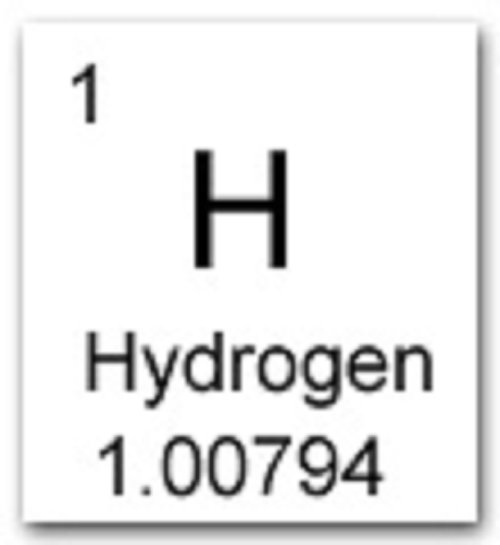
Isotopes are discussed more in depth later in this module. Since isotopes have a different number of neutrons, their mass numbers and atomic masses differ from those listed in the periodic table. The atomic mass is typically listed in the periodic table below the element's name. The atomic mass of Hydrogen is 1.00797 amu and the atomic mass of Carbon is 12.011 amu. The mass number is approximately equal to the atomic mass, which is the mass of a single atom of a element measured in atomic mass units (amu). For example the mass number of a regular carbon atom is 12, since a carbon atom has 6 protons and 6 neutrons in its nuclus.
#Hydrogen atomic mass plus#
In words, the mass number is the number of neutrons in an atom of a specific element plus the number of protons in an atom of that element. Isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects their mass number. Mass NumberĪll atoms have a mass number which is derived as follows: By definition, an atom of carbon with six neutrons, carbon-12, has an atomic mass of 12 amu. The atomic mass of a single atom is simply its total mass and is typically expressed in atomic mass units or amu. The atomic number of an element never changes, meaning that the number of protons in the nucleus of every atom in an element is always the same. A property closely related to an atom’s mass number is its atomic mass. Oxygen atoms contain 8 protons and have an atomic number of 8. All carbon atoms, and only carbon atoms, contain six protons and have an atomic number of 6. What is a weighted average Interactive popup. For example, all hydrogen atoms, and only hydrogen atoms, contain one proton and have an atomic number of 1. Oxygen equal to 16.00 has been accepted by the International Committee on Atomic Weights as the standard, and the column referred to hydrogen equal to 1 has. This hydrogen isotope has an atomic mass of 3amu or 1 proton and 2 neutrons. In other words, each element has a unique number that identifies how many protons are in one atom of that element. The number of protons in the nucleus of an atom determines an element's atomic number. Define and determine the mass number of an atom.Define and determine the atomic number of an atom. This and a few similar cases suggested the idea, that, if the atomic weights wrere not even multiples of the received hydrogen atom, they might be mul.Hydrogen is the first element on the periodic table, and is the most basic element. After reading this section you will be able to do the following: Hydrogens atomic mass is 1.008amu, or 1.008 grams mole.
When an organism dies, it stops taking in carbon-14, so the ratio of carbon-14 to carbon-12 in its remains, such as fossilized bones, will decline as carbon-14 decays gradually to nitrogen-14 2 ^2 2 squared.

As animals eat the plants, or eat other animals that ate plants, the concentrations of carbon-14 in their bodies will also match the atmospheric concentration. As plants pull carbon dioxide from the air to make sugars, the relative amount of carbon-14 in their tissues will be equal to the concentration of carbon-14 in the atmosphere. These forms of carbon are found in the atmosphere in relatively constant proportions, with carbon-12 as the major form at about 99%, carbon-13 as a minor form at about 1%, and carbon-14 present only in tiny amounts 1 ^1 1 start superscript, 1, end superscript. For example, carbon is normally present in the atmosphere in the form of gases like carbon dioxide, and it exists in three isotopic forms: carbon-12 and carbon-13, which are stable, and carbon-14, which is radioactive.


 0 kommentar(er)
0 kommentar(er)
